A tablet is a pharmaceutical dosage form. It comprises a mixture of active substances and excipients, usually in powder form, pressed or compacted into a solid. The compressed tablet is the most popular dosage form in use today. About two-thirds of all prescriptions are dispensed as solid dosage forms, and half of these are tablets. A tablet can be formulated to deliver an accurate dosage to a specific site; it is usually taken orally, but can be administered sublingually, buccally, rectally or intravaginally. Medicinal tablets were originally made in the shape of a disk of whatever color their components determined, but are now made in many shapes and colors to help distinguish different medicines. Tablets are often stamped with symbols, letters, and numbers, which enable them to be identified. Sizes of tablets to be swallowed range from a few millimeters to about a centimeter. Some tablets are in the shape of capsules, and are called "caplets".
According to BP tablet may be classified as follow
1. Uncoated tablets
2. Coated tablets
3. Effervescent tablets
4. Soluble tablets
5. Dispersible tablets
6. Gastroresistant tablets
7. Modified release tablets
8. Tablets use in the mouth
Tablet is solid dosages form in which active ingredient and excipient remain in compact form for presence of various exipient.
A tablet is usually a compressed preparation that contains:
5-10% of the drug (active substance);
80% of fillers, disintegrants, lubricants, glidants, and binders; and
10% of compounds which ensure easy disintegration, disaggregation, and dissolution of the tablet in the stomach or the intestine.
Exipients used in tablet formulation are as follow.
Fillers and diluents
Fillers fill out the size of a tablet or capsule, making it practical to produce and convenient for the consumer to use. By increasing the bulk volume, the fillers make it possible for the final product to have the proper volume for patient handling. For Examples: Maize starch, Calcium hydrogen phosphate, pregelatinized starch, lactose.
Binders
Binders hold the ingredients in a tablet together. Binders ensure that tablets and granules can be formed with required mechanical strength, and give volume to low active dose tablets.
For Examples: Starch, Na-alginate, maize starch, pregelatinized starch, povidone K30
Disintegrants
Disintegrants expand and dissolve when wet causing the tablet to break apart in the digestive tract, releasing the active ingredients for absorption. Disintegrant types include:
- Water uptake facilitators
- Tablet rupture promoters
They ensure that when the tablet is in contact with water, it rapidly breaks down into smaller fragments, thereby facilitating dissolution. Examples of disintegrants include:
- Crosslinked polymers: crosslinkedpolyvinylpyrrolidone (crospovidone), crosslinked sodium carboxymethyl cellulose (croscarmellose sodium).
Lubricants
Lubricants prevent ingredients from clumping together and from sticking to the tablet punches or capsule filling machine. Lubricants also ensure that tablet formation and ejection can occur with low friction between the solid and die wall.
Common minerals like talc or silica, and fats, e.g. vegetable stearin, magnesium stearate or stearic acid are the most frequently used lubricants in tablets or hard gelatin capsules.
Glidants
Glidants are used to promote powder flow by reducing interparticle friction and cohesion. These are used in combination with lubricants as they have no ability to reduce die wall friction. Examples include fumed silica, talc, and magnesium carbonate.
Flavors
Flavors are used to mask unpleasant tasting active ingredients and improve the likelihood that the patient will complete a course of medication. Flavorings may be natural (e.g. fruit extract) or artificial.
To improve drug flavors;
a bitter product - mint, cherry or anise may be used
a salty product - peach, apricot or liquorices may be used
a sour product - raspberry or liquorices may be used
an excessively sweet product - vanilla may be used
Colorants
Coloring agents are added to improve the appearance of a formulation. Color consistency is important as it allows easy identification of a medication
MANUFACTURING METHODS:
In the tablet-pressing process, it is important that all ingredients be dry, powdered, and of uniform grain size as much as possible. The main guideline in manufacture is to ensure that the appropriate amount of active ingredient is equal in each tablet so ingredients should be well-mixed. Compressed tablets are exerted to great pressure in order to compact the material. If a sufficiently homogenous mix of the components cannot be obtained with simple mixing, the ingredients must be granulated prior to compression to assure an even distribution of the active compound in the final tablet. Two basic techniques are used to prepare powders for granulation into a tablet: wet granulation and dry granulation.
Powders that can be mixed well do not require granulation and can be compressed into tablets through Direct Compression
Dispensing Unit
The manufacture unit sends a demand paper according to their need to the warehouse. Central Dispensing Unit weights the required amount of API and excipents. Weight of row material and excipients depends on the basis of potency.
The manufacturing tablet dosage form by two methods such as
1. Wet Granulation (most products)
2. Direct Compression
WET GRANULATION
Wet Granulation is a process of size enlargement whereby small particles are gathered into larger permanent aggregates in which the original particles can still be identified. Granulation usually refers to processes whereby agglomerates with sizes ranging from 0.1 to 2.0 mm are produced. The most important reasons for a granulation step prior to tableting are to:
▪ Improve the flow properties of the mix and hence the uniformity of the dose
▪ Prevent segregation of the ingredients
▪ Improve the compression characteristics of the tablet mixture
▪ Reduce dust during handling
The flow ability of the tablet mixture improves because the granules are larger and more spherical than the primary particles. Larger particles usually flow better than small particles (e.g. compare the flow ability of crystal sugar with powder sugar). In the hopper of tablet machines, small particles tend to segregate from the larger ones because of the vibration of the machine. This causes higher concentrations of small particles at the bottom of the hopper. After granulation all particles are bound tight in the right amount in the granules, which prevents segregation of the small particles
Process Flow Chart
(Wet granulation method)
Equipments used in wet granulation method:
1. Electronic Balance
2. Sieve
3. Rapid Mass Granulator (RMG)
4. Multimill
5. Fluid Bed Dryer
6. Double Cone Blender
7. Vat for the preparation of granulating fluid
DIRECT COMPRESSION
In the direct compression method, directly compressible filler (also called a filler-binder) is blended with the active(s), a lubricant and a disintegrating agent. Such free flowing directly compressible fillers make direct compression possible and practical. These include anhydrous lactose, unmilleddicalcium phosphate dihydrate, microcrystalline cellulose (e.g., Avicel PH 101), and modified (spray processed) lactose (e.g., Ludipress). Modified starch, e.g. Starch 1500 flows better and compresses better than original starch, but are not as effective as other materials as the sole filler-binder. Generally, Starch 1500 is used as a component of a direct compression filler system, most likely for its disintegrating property, i.e., as a more compactible and better flowing substitute for starch. Certain materials like mannitol, sorbitol and modified sucrose are particularly useful in formulating direct compression chewable tablets.
Direct compression method can be classified as
Ø a) Direct Compression with direct compressible materials and
Ø b) Direct Compression by Slugging method
Process flow chart
(Direct Compression with direct compressible materials)
Equipments used in direct compression method:
1. Electronic Balance
2. Sieve
3. Double cone blender
4. Rotary Press
Parameters checked prior to tablet manufacturing:
1. Proper cleanliness of all machines and room
2. Check the room temperature humidity and air pressure
3. Proper mixing to distribute all ingredient uniformity
4. Check LOD
5. Proper selection of die and punch
6. Compression pressure speed of the machine
Problem in Tablet manufacturing Process
An ideal tablet should be free from any visual defect or functional defect. The advancements and innovations in tablet manufacture have not decreased the problems, often encountered in the production, instead have increased the problems, mainly because of the complexities of tablet presses; and/or the greater demands of quality. An industrial pharmacist usually encounters number of problems during manufacturing. Majority of visual defects are due to inadequate fines or inadequate moisture in the granules ready for compression or due to faulty machine setting. Functional defects are due to faulty formulation. Solving many of the manufacturing problems requires an in–depth knowledge of granulation processing and tablet presses, and is acquired only through an exhaustive study and a rich experience. Here, we will discuss the imperfections found in tablets along–with their causes and related remedies. The imperfections are known as: ‘VISUAL DEFECTS’ and they are either related to imperfections in any one or more of the following factors:
I. Tableting Process
II. Excipient
III. Machine
1) Defects related to Tableting Process
a) i) Capping: Capping is the term used, when the upper or lower segment of the tablet separates horizontally, either partially or completely from the main body of a tablet and comes off as a cap, during ejection from the tablet press, or during subsequent handling. Reason: Capping is usually due to the air–entrapment in a compact during compression, and subsequent expansion of tablet on ejection of a tablet from a die.
Remedies: Moisten the granules properly, add hydroscopic substance e.g. sorbitol.
b) ii) Lamination: ‘Lamination’ is the separation of a tablet into two or more distinct horizontal layers.
Reason: Air–entrapment during compression and subsequent release on ejection. The condition is exaggerated by higher speed of turret.
Remedies: Modify mixing process, use less amount of lubricant.
c) iii) Cracking: Small, fine cracks observed on the upper and lower central surface of tablets, or very rarely on the sidewall are referred to as ‘Cracks’.
Reason: It is observed as a result of rapid expansion of tablets, especially when deep concave punches are used.
Remedies: Dry the granules sufficiently.
2) Defects related to Excipient are as follows:
a) CHIPPING: ‘Chipping’ is defined as the breaking of tablet edges, while the tablet leaves the press or during subsequent handling and coating operations.
Reason: Incorrect machine settings, specially mis-set ejection take-off.
Reason: Incorrect machine settings, specially mis-set ejection take-off.
b) STICKING / FILMING: ‘Sticking’ refers to the tablet material adhering to the die wall.
Filming is a slow form of sticking and is largely due to excess moisture in the granulation.
Reason: Improperly dried or improperly lubricated granules.
Remedies:
• Partial or complete substitution of low melting point components with high melting point materials in the formula
• Proper drying of the granules to remove excessive moisture
c) PICKING: ‘Picking’ is the term used when a small amount of material from a tablet is sticking to and being removed off from the tablet-surface by a punch face. The problem is more prevalent on the upper punch faces than on the lower ones. The problem worsens; tablets are repeatedly manufactured in this station of tooling because of thmore and more material getting added to the already stuck material on the punch face.
Reason: Picking is of particular concern when punch tips have engraving or embossing letters, as well as the granular material is improperly dried.
Remedies:
i) Lettering should be designed as large as possible, even the tablet size can be increased by reformulation.
ii) Colloidal silica can be added as polishing agent to formula
iii) Using additional binder to increase cohesiveness of granules and thereby causing decreased adherence
iv) Plating of punch faces with a chromium material to obtain smooth face which is non-adherent
d) BINDING: ‘Binding’ in the die is the term used when the tablets adhere, seize or tear in the die. A film is formed in the die and ejection of tablet is hindered. With excessive binding, the tablet sides are cracked and it may crumble apart.
Reason: Binding is usually due to excessive amount of moisture in granules, lack of lubrication and/or use of worn dies.
Remedies: Reduce the amount of binder or use a different type of binder.
3) Defect related to Machine
Ø ‘Double Impression’ involves only those punches, which have a monogram or other engraving on them.
Reason: At the moment of compression, the tablet receives the imprint of the punch. Now, on some machines, the lower punch freely drops and travels uncontrolled for a short distance before riding up the ejection cam to push the tablet out of the die, now during this free travel, the punch rotates and at this point, the punch may make a new impression on the bottom of the tablet, resulting in ‘Double Impression’. If the upper punch is uncontrolled, it can rotate during the short travel to the final compression stage and create a double impression.
Remedy: Using anti-turning devices
4) The defect related to more than one factor:
Ø Mottling: It is either due to any one or more of these factors: Due to a colored drug, which has different color than the rest of the granular material? (Excipient- related); improper mixing of granular material (Process-related); dirt in the granular material or on punch faces; oil spots by using oily lubricant.
Remedies:
• By using bright coloring agent that will mask all the color variations of the ingredients
• Proper drying by reducing the drying temperature
• Colored adhesive gel solutions must be added when they are hot to much cooler powder mixtures to avoid precipitation
• It is better to incorporate fine powder adhesives like acacia and tragacanth into product before adding the granulating fluid
• By changing the solvent system or binder system
• Grinding to small particle size
Tablet coating unit
Oral tablets coated with a layer of sugar or film are called coated tablet. The application of coating to tablets, which is an additional step in the manufacturing process, increases the cost of the product. Again the coat must be dissolved before disintegration and dissolution of the tablet. Therefore, the advantage to coat a tablet is usually based on one or more of the following objectives:
a) To mask the bad taste, order, or color of the drug.
b) To protect moisture sensitive drugs from moisture.
c) To provide physical and chemical protection of the drug.
d) To protect the drug from the gastric environment of the stomach with an acid resistant enteric coating.
e) To control the release of the drug from the tablet.
f) To incorporate another drug or adjuvant in the coating to avoid chemical incompatibilities or to provide
sequential drug release.
g) To improve the pharmaceutical elegance by use of special color or contrasting printing.
Coating process:
The following three processes are employed for tablet coating:
1. Film coating
a) Aqueous film coating
b) Organic film coating
2. Enteric coating
a) Liquid enteric coating
b) Powder enteric coating
1. Film coating:
This process involves the deposition of a thin polymeric film onto tablets from solutions that are organic solvent based or water based. Some substances used for film coating in ACI are mentioned bellow:
a) Kollicoat (coating agent)
b) Titanium dioxide (increase shining)
c) Purified talc (increase flow property)
d) Allura red lake (coloring agent)
e) Opadry II (coating agent)
f) Opadry OY-S (coating agent)
g) Opaglos (plasticizer, shining agent)
a) Aqueous Film coating process
Preparation of coating solution
Coating process
b) Organic film coating process:
I. Preparation of pre-coating solution.
Here plasticizer and suitable organic solvents are mixed well and initially sprayed onto the tablets to provide a water resistant thin layer.
II. Preparation of final coating solution.
Here film coating agent and suitable organic solvent is used for spraying the tablets.
Enteric coating:
An enteric coat is usually a special film coat designed to resist its destruction into gastric fluid and to disrupt or dissolved in the small intestine.
Materials used in enteric coating:
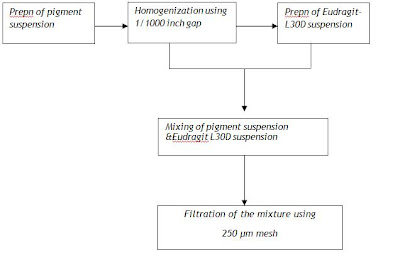
· Eudragit L30D
· Triethyl citrate
· Talcum powder
· Titanium dioxide
· Red iron oxide
· Tween-80
Preparation of coating solution
Coating process
- Coating pan is cleaned and free from any tablet of previous.
- Open the compressed air line. Turn on the atomizer for checking & ensure that both spray guns blow air properly.
- Load the pan with tablets. Preheat the tablets at 40°C±2°C inlet air for approximately 15 minutes. Jog pan every minute for 10 seconds.
- Fix the spray guns 6" apart from each other, 10" above & perpendicular to the bed.
- Connect one end of silicon tube to spray guns and other end to the solution tank through the peristaltic motor.
- Connect the compressed air line to the solution tank and open the line for stirring the coating solution gently.
- Jog the pan for few times while the inlet & outlet air temperature attain the desire level. Inlet temperature should 37-38°C.
- Switch on the pan, atomizer & peristaltic pump respectively for coating tablets.
- Set the RPM of peristaltic pump. (22)
- Set the atomizing air pressure. (2-2.5 Kg/cm2)
- Set the RPM of the pan at 5-6. (target 5 ½)
- Continue spray until all suspensions have been used.
- After completion of coating stop peristaltic pump & atomizer respectively. Then dry the tablets for 15-20 minutes with tablet tumbling at a slow pan speed.
- Stop blower, exhaust, heater and pan respectively.
- Discharge tablets.
Coating defects
Here is a list of common defects associated with coated tablets and some likely causes.
Problem: Tablet is rough and has poor engraving definition, a result of rapid spraying with a coating suspension containing 15% solids.
Solution: Spray under the same conditions but with 12% solids. Typically, the higher the solids content, the rougher the coating.
Ø ii) EDGEWEAR
Problem: The shape of tablet causes it to slide in the pan, thus inviting abrasion on the leading edge.
Solution: Add extra slide bars and baffles, which ensure that the tablet rolls in a way that enables uniform coating.
Ø iii) UNIFORMITY
Problem: Pill lacks uniform coating, especially noticeable in the early stages of the process. The problem persists throughout the process, with variation obscured by color development.
Solution: The variation is a function of the mixing and frequency of presentation in the spray zone. Enlarge the spray zone and spray more tablets at each pass; tablets will receive more applications and variation will be reduced.
Ø iv) BRIDGING
Problem: Tablet logo is lost or obscured by bridging. The logo appears filled in, but cleaving the tablet across the logo shows a void, indicating that the film did not adhere to the engraving.
Solution: Increase plasticizer to improve flexibility or modify the resin system. HPC and PVP improve adhesion when replacing at least 20% of the HMPC.
Ø v) MOTTLING
Problem: Poor dispersion of pigments or poor atomization of coating suspension is often the culprit in dye coating.
Solution: Developed a technique to immobilize dye in the coating suspension, thus replacing lakes, which are insoluble pigments.
Ø vi) SPLITTING
Problem: Splitting on pills is caused by a film that is weak and brittle due to excessive pigment loading.
Solution: Modify the coating suspension.
Ø vii) FADING
Problem: Light grey tablets faded due to small quantity of color. When lake concentration is very low, the tendency to fade is high, especially in the presence of PEG.
Solution: Coat in a film without PEG, which has a deleterious effect on light stability of all lakes.
Ø viii) PICKING
Problem: Tablet is over-wetted due to blinding of the bag in the column of a Glatt Wurster unit. Because the core is waxy and less moisture sensitive, the film picks from one tablet to another.
Solution: Remove the bag filter.
Ø ix) CHIPPING
Problem: The edge of tablet is raised (flashing due to worn punches) and the film coating is weak.
Solution: Replace 20% of 5cP HPMC with 15cP HPMC. This increases the tensile strength and toughens the film enough to hold the weak edges. Reducing pigment might achieve the same result, but replacing worn punches and eliminating the flashing is the best solution.
Ø x) BLISTERING
Problem: The most common problem with aqueous film coating, tablet becomes over-wet (not too over-wet), thus activating a moisture-sensitive spot in the core. Swelling begins, thus raising the point and causing excessive wear that degrades the film. The film does not break, but the tablet is disfigured, producing a scab on the tablet.
Solution: Raise the bed temperature or process air volume or reduce the spray rate.
Ø xi) EROSION
Problem: Tablet is friable despite having adequate hardness. The surface erodes during the warm-up period in the pan. The coating process develops properly, but the tablet is ruined by erosion. Edge erosion is similar to chipping.
Solution: Reduce pan speed or increase the strength of the film deposited. Generally, once a film covers the edges, pan speed can be increased in the later stages of coating. This is desirable to reduce variation.
xii) TWINNING
Problem: Caplets twin when over-wetted.
Solution: Keep the spray rate within the limits of the drying capacity of the equipment and increase the speed of the pan. Capsule-shaped tablets should be designed without straight edges. Any tablet with a flat surface will tend to twin.
References:
1. Wikipedia
2. The Theory and practice of Industrial Pharmacy,
(Leon. Lachman, H.A. Lieberman, J.L. Kanig)
3. sensientpharma.com
4. Dictionary of Pharmaceutical Technology
(Dr. Abu Jamil Ferdous, Dr. Reza Ul-Jamil)
5. Modern Pharmaceutics basic principles and systems,
(Florence and Siepmann)
6. pharmainfo.net
1. Wikipedia
2. The Theory and practice of Industrial Pharmacy,
(Leon. Lachman, H.A. Lieberman, J.L. Kanig)
3. sensientpharma.com
4. Dictionary of Pharmaceutical Technology
(Dr. Abu Jamil Ferdous, Dr. Reza Ul-Jamil)
5. Modern Pharmaceutics basic principles and systems,
(Florence and Siepmann)
6. pharmainfo.net


























Thanks for sharing this wonderful informative post, it has everything you need to know about pharmaceutical tablets.
ReplyDeleteWe are a Professional Turnkey Project Management company. We supply plants and machinery for Lube Oil Blending Plant, Grease Manufacturing Plant, and various other Petrochemical Industries. On the other hand, , we have got expertise in different plants such as Cooking Gas Cylinder mfg. Blow Moulding plant, etc. We have supplied plants and Machinery around the globe such as Africa, Asia, the Caribbean, Central America, Europe, North America, etc Lubricant filling machine
ReplyDeleteNice articles and your information valuable and good articles thank for the sharing information lubricant blending plant
ReplyDeleteA Oil Blending Plant is a specialized facility where various lubricating oils are produced through the blending and mixing of base oils with additives.
ReplyDelete